Developing technology that helps people save lives
Our first product, AIR, gives birth attendants real-time resuscitation feedback for effective newborn ventilation training.
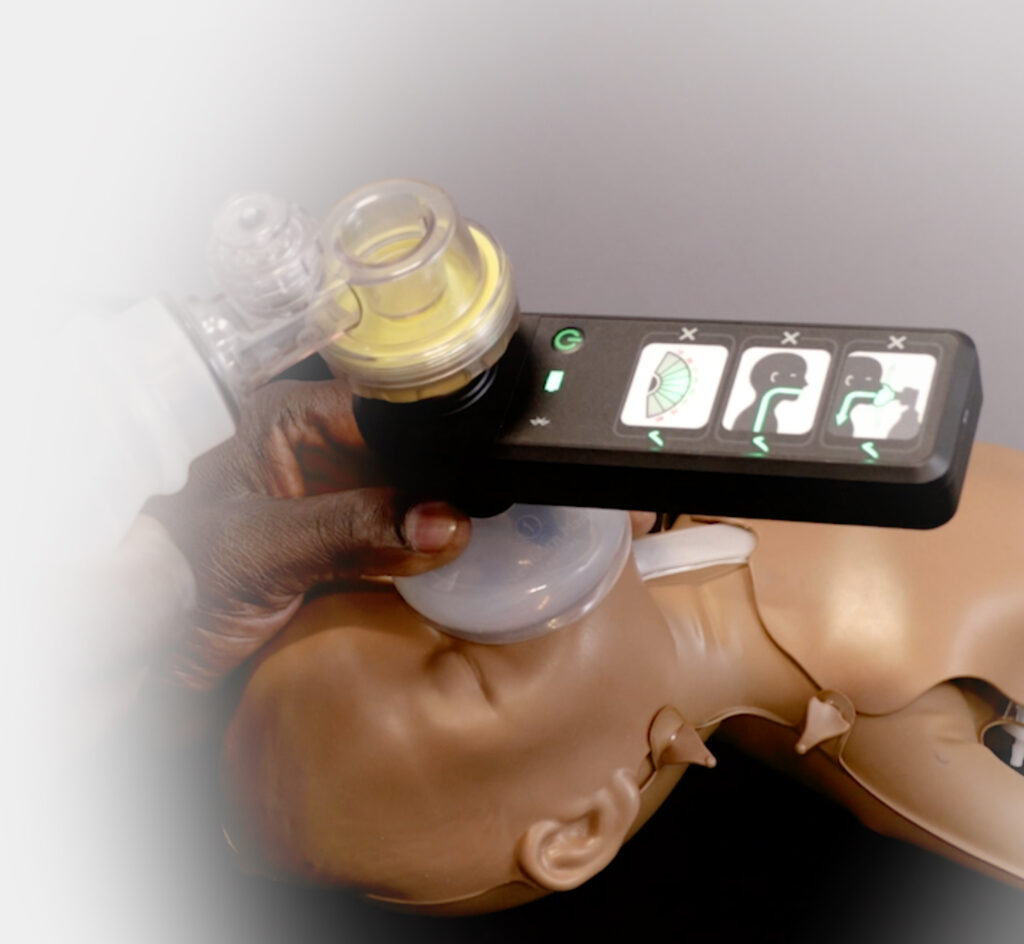
AIR – Augmented Infant Resuscitation
Giving birth attendants needed training for confidence to deliver the first breath of life
The first 60 seconds of life are crucial
For babies born not breathing, every 30 second delay to achieve effective ventilation increases the risk of death by 16%1
20% of current standard training doesn’t stick
1 in 5 of the best-trained health workers will fail to perform effective ventilation immediately after training2
AIR helps retain resuscitation skills
AIR is a patented resuscitation training aid that provides objective, real-time feedback so healthcare workers can achieve effective ventilation sooner and maintain ventilation for longer3
- Ersdal HL, Mduma E, Svensen E, Perlman JM. Early initiation of basic resuscitation interventions including face mask ventilation may reduce birth asphyxia related mortality in low-income countries: A prospective descriptive observational study. Resuscitation 2012;83:869-73.
- Patel J, Posencheg M, Ades A. Proficiency and retention of neonatal resuscitation skills by pediatric residents. Pediatrics 2012;130:515-21.
- Data S, Nelson BD, Cedrone K, et al. Real-Time Digital Feedback Device and Simulated Newborn Ventilation Quality. Pediatrics. 2023;152(5):e2022060599.
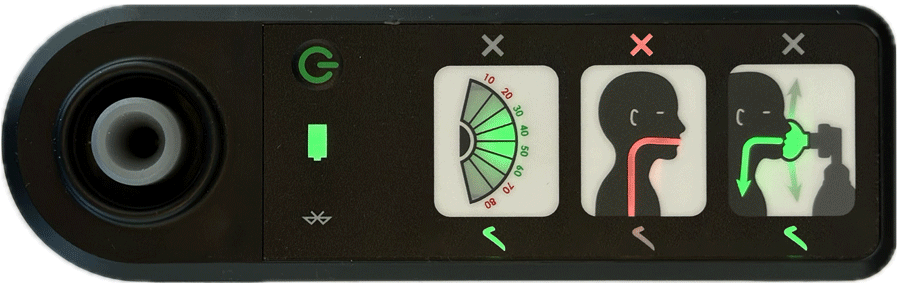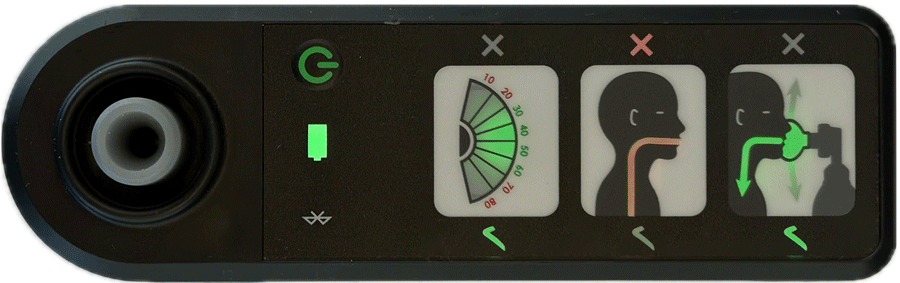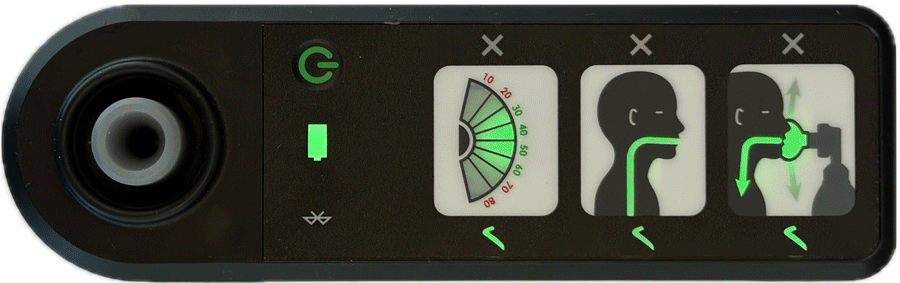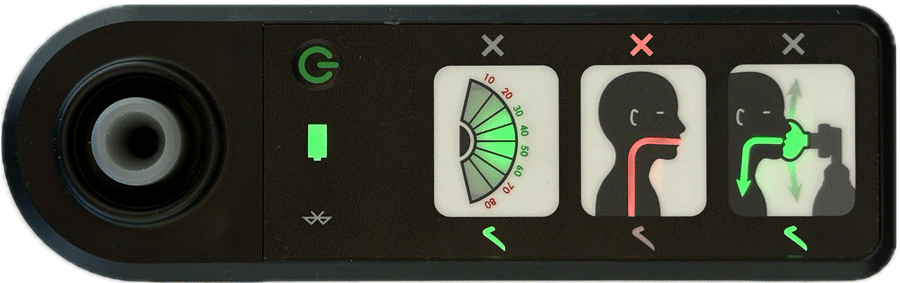
AIR is an affordable Bag Valve Mask (BVM) solution that improves neonatal resuscitation training
We developed AIR’s patented technology to help birth attendants develop the skills and confidence in achieving and maintaining effective ventilation within the first minute of life.
Real-time feedback is provided by four indicators showing:
- effective face-mask seal
- rate of breathing
- airway blockage
- gentleness of breaths delivered
Indications of effective ventilation are intuitive and don’t rely on language to provide feedback, making it a perfect training aid in all settings.
AIR is Bluetooth enabled and connects to an app for mobile use. The AIR Web Dashboard provides anonymized, grouped data so health delivery organizations can monitor the frequency and quality of practice allowing for evolving feedback in order to improve training programs.
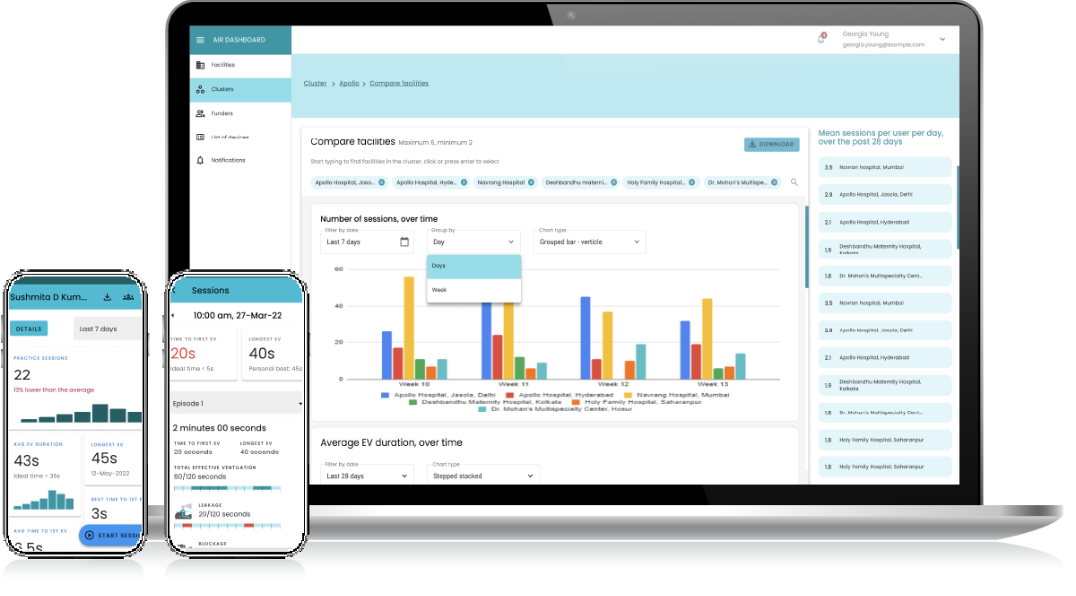
Learn how AIR helps birth attendants build their confidence in neonatal resuscitation
Using AIR leads to better infant resuscitation training outcomes
2x
faster
Effective ventilation is achieved twice as quickly with AIR1
1.5x
longer
Effective ventilation is maintained longer with AIR1
Quick
airway assessment
AIR feedback helps aid in faster and more accurate assessment of airway conditions1
- Results from: Real-time digital feedback device and simulated newborn ventilation quality. Pediatrics (2023): 152(5).
Do you want AIR for your institution or practice?
The EB Innovations Team
Our team is dedicated to developing technologies that address world-wide health problems, so we can help more people save lives.
Founding Board Members
Dr. Santorino Data, MBChB, MMed
Founder & Board Member
- Uganda country manager: Consortium for Affordable Medical Technologies (CAMTech)
- Senior Lecturer of Pediatrics: Mbarara University of Science and Technology
- Master trainer: Helping Babies Breathe & Essential Care for Every Baby
Dr. Data has spent years training people to help babies breathe better, from frontline workers in Uganda and Ethiopia, and through work with organizations like USAID, Save the Children International, Laerdal Foundation, MCHIP, American Academy of Pediatrics, and JHPIEGO.
Dr. Data has seen first hand some of the challenges in training people to achieve and maintain effective ventilation, and has used that experience as a co-inventor of the AIR platform.
His passion for providing accessible medical technology and training for healthcare providers around the world is what fuels his drive to continue developing AIR.

Dr. Kristian Olson, MD, MPH, DTM&H
Founder and Board Member
- Vice President of Design Impact: Mass General Brigham
- Pediatrician and Internist: Massachusetts General Hospital
- Core Clinical Educator: Massachusetts General Hospital
- Associate Professor: Harvard University
Dr. Kristian Olson has worked in Boston, Thailand, Darfur, Indonesia, Cambodia, Ethiopia, Uganda, and India, to deliver healthcare, guide the design and development of healthcare solutions, and train healthcare providers.
Dr. Olson is a co-inventor of the AIR platform. He is a serial innovator with a passion for human-centered design in healthcare and creating affordable medical technologies.
As a Pediatrician and Internal Medicine Physician, an international trainer of the Helping Babies Breathe since its launch, and a newborn resuscitation trainer since 2003, Dr. Olson immediately saw the utility of AIR to enhance training programs across the world.

Kevin Cedrone, PhD, Mechanical Engineering
Founder & Board Member
- Co-founder and Head of R&D, Lumafield Inc.
Kevin Cedrone holds both a Masters and PhD in mechanical engineering, both from the Massachusetts Institute of Technology.
He researched the efficiency and emissions of automotive combustion engines. As a postdoctoral research associate at the Plasma Science and Fusion Center at MIT, he researched novel systems to convert natural gas to liquid hydrocarbon fuels.
Kevin is a co-inventor of the AIR platform. He has translated his passion for global health and development into several award-winning innovative medical devices.
Executive Team

Jim Miller, JD
CEO
Mr. Miller currently consults at Mass General Brigham where he advises and accelerated novel ideas that hold promise to make healthcare more user-friendly. Jim is the co-founder of two high impact medical device companies. Previously, he was Chief Global Mentor for Mass Challenge, Executive in Residence at Partners (MGB) Healthcare Innovation, and Director of the Massachusetts Environmental Crimes Strikeforce. He has served as a Senior Advisor to USAID and participated in founding the Private Capital Group-Africa (PCGA) to encourage innovative partnerships between private capital and local enterprise in sub-Saharan Africa. He is a serial entrepreneur, having co-founded two national retail chains (Bertucci’s and FiRE+iCE Restaurants) and subsequently two medical device enterprises, all of which resulted in successful exits including 3 IPOs.

Doug Marsden
CTO
Doug Marsden is the CTO and co-founder of Eleven, a human centered design studio that integrates research, design, strategy, and engineering under one roof. He is also a founder of Healthcare Innovation Partners. He has over 30 years of experience driving business growth for new technology.
Advisory Board

Steven Ringer
MD, PhD, Neonatology
Children’s Hospital at Dartmouth-Hitchcock
Steven Ringer is a Board Certified Neonatologist and Professor of Pediatrics at the Geisel School of Medicine at Dartmouth University. He has over 30 years of Neonatology experience. He has served in national and international leadership positions in his field including serving on the International Liaison Committee on Resuscitation. He has advised the successful development of several medical products serving the neonatal space for deployment in both Low and Middle Income Countries as well a High Income Countries.

Ray DeSabato
Ray is a veteran entrepreneur with over 30 years of experience building technology companies. He is currently the CEO and Co-founder of Chorus, a healthcare technology company. Previously he served as the CEO of Kuebix, a supply chain SaaS enterprise. He raised over $35M through institutional and strategic investors to build a company of over 100 people that served 20,000 companies. Kuebix was sold in January of 2020 to Trimble Inc and generated an 8x return for investors. Before Kuebix, Ray achieved similar results for technology companies like Geo-Tech Polymers and SynQor. Before those entrepreneurial endeavors, Ray spent 5 years at American Power Conversion in various leadership position which included worldwide operations of a $350M business Unit.
How We Got Here and Where We’re Going

EB Innovations is the result of collaborations with professionals around the world that are passionate about developing technology that help save lives.
In 2012, Dr. Santorino Data, an experienced global trainer of newborn resuscitation, stood before a motivated group of clinicians, designers, and engineers in Boston with a request. “Is there a technology that can help us teach birth attendants to ventilate more effectively?”
A serendipitous meeting occurred. Dr. Kevin Cedrone, a mechanical engineer, Mr. Craig Mielcarz, an electrical engineer, and Dr. Kristian Olson, a Pediatrician and Internal Medicine Physician all converged to answer Dr. Data’ call.
Kevin, a Postdoctoral student at the time said, “I have sensors in the trash in my lab across the river [at MIT] that I think might give us an idea of how to solve this.” After a sleepless night, the team presented a rudimentary but functional prototype to the event judges on the following day – and won the award for most innovative solution!
After a decade of human-centered design, several multinational trials and 6 iterations of the device, the AIR device is now ready for scaled release. So what’s next?
AIR helps providers achieve faster and longer lasting effective ventilation while training neonatal resuscitation. Following resuscitation training, continued use of AIR for ventilation training was demonstrated in Kenya and India to cause continued ventilation skills growth. We hope to expand the use of AIR to clinical settings, where providers can save more lives during an emergency.
At the same time, we want to expand the application of AIR beyond neonatal resuscitation and into adult resuscitation.
Because the AIR device is small, reusable, and cost-effective, we envision a future where every ambulance and crash cart has an AIR device.
Milestones
2012
It started with a CAMTech problem solving session, and some spare parts in an MIT lab
2014
A technology acceptance and feasibility study in Uganda confirms the viability of the AIR prototype
2017
Independent assessment of accuracy establishes AIR as extremely accurate
2020
Cost effectiveness study shows that AIR is extremely cost-effective to implement for training programs
2023
A study published in Pediatrics showed that birth attendants achieved faster and longer lasting effective ventilation with the AIR device
Looking forward
We are now working to disseminate AIR broadly to assist in skill retention of birth attendants; refining the adult training augmentation tool; and are working on development of a clinical-use tool to be available wherever urgent bag-valve-mask ventilation is required

Our Supporters









Selected Publications & Trials
2023: Real-time digital feedback device and simulated newborn ventilation quality in training
- 2x faster effective ventilation
- 1.5x more sustained effective ventilation
- Faster and more accurate airway assessment
Real-time digital feedback device and simulated newborn ventilation quality. Pediatrics (2023): 152(5).
2020: Cost Effectiveness Study
- Implementation of AIR results in an addition cost of less than $25 per DALY averted
- Per hospital, AIR adds extra $656 to initial training costs
- AIR averts ~26.84 years of disability in the cohort of children born in the first year
Cost effectiveness of a novel device for improving resuscitation of apneic newborns. BMC Pediatrics (2020): 20(46)
2017: Independent Evaluation of Accuracy
- No deleterious effect on breath parameters
- 100% detection of leaks and obstructions on manikins
- Displayed the correct indicators 73.5% on simulated clinical scenarios while using the training firmware
Evaluation of the augmented infant resuscitator: A monitoring device for neonatal bag-valve-mask resuscitation. Anesth Analg (2018): 126(3).
2014: Acceptability and feasibility study
- Confirms the viability of the AIR prototype
- Users were receptive to the idea of live feedback during instruction, simulation, and clinical practice
Augmented infant resuscitation (AIR) device to improve management of intrapartum related deaths (“birth asphyxia”). Appropriate Healthcare Technologies for Low Resource Settings (AHT 2014).
News and Announcements
- April 25, 2024: An international dissemination meeting was hosted by USAID’s Momentum. The exciting pre-publication results of the 2023-2024 randomized step-wedge trial of the AIR platform in India and Kenya was presented. 60 participants attended the virtual meeting from Canada, India, Kenya, Norway, Uganda, and the United States. (The Momentum project works with USAID partner countries to tackle unique, country-specific challenges, while ensuring interventions have the best possible impact and reach.)

Contact Us
Disclaimer
This is technology is developed and is commercially available for simulated training purposes only. It has not been evaluated by any regulatory agencies for safety or efficacy in clinical-use settings.
Neither EB Innovations LLC nor any manager, officer, member or employee of EB Innovations LLC will be responsible for any errors or omissions in the contents of this site, however caused.
